complete the mechanism for an azo coupling by drawing in the curved arrows where needed.
Diazonium Salts From Amines, And Reactions Of Diazonium Salts
Today let's talk about a set of reactions of aromatic amines, that variously are catalogued under "amines" and "aromatic compounds", depending on the textbook.
It involves converting an aromatic amino group (NH2) into a tremendously good leaving group (N2) which can then be replaced by various nucleophiles. However, N2 issuch a good leaving group that the method only works well for aromatic amines; alkyl ("aliphatic") amines tend to lose N2too rapidly, making the method much less useful in that case.
Table of Contents
- Formation of Diazonium Salts From Aromatic Amines
- Reactions of Diazonium Salts: Sandmeyer And Other Reactions
- Mechanism: Formation of Diazonium Ions
- Bonus Reaction: Diazo Coupling
- Quiz Yourself!
- (Advanced) References and Further Reading
1. Formation of Diazonium Salts From Aromatic Amines
Here's the process. Treatment of an aromatic amine with nitrous acid (or sodium nitrite, which is converted to nitrous acid in the presence of acid) in the presence of a strong acid like HCl results in the loss of H2O and the formation of a new N-N triple bond. The resulting species is called a "diazonium ion":
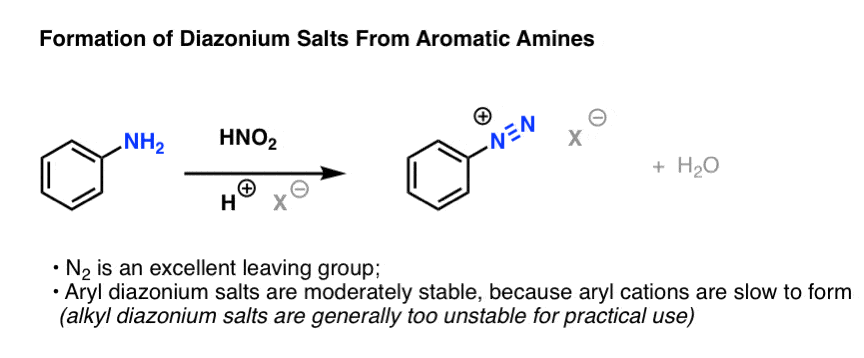
(How does it work? We'll go through the mechanism at the bottom of the post).
2. Reactions of Diazonium Salts: Overview
So why does it matter?
It matters because the resulting diazonium salts can be transformed into all kinds of useful functional groups. Rather than describe everything in words, first let's just show 7 examples with a diagram:
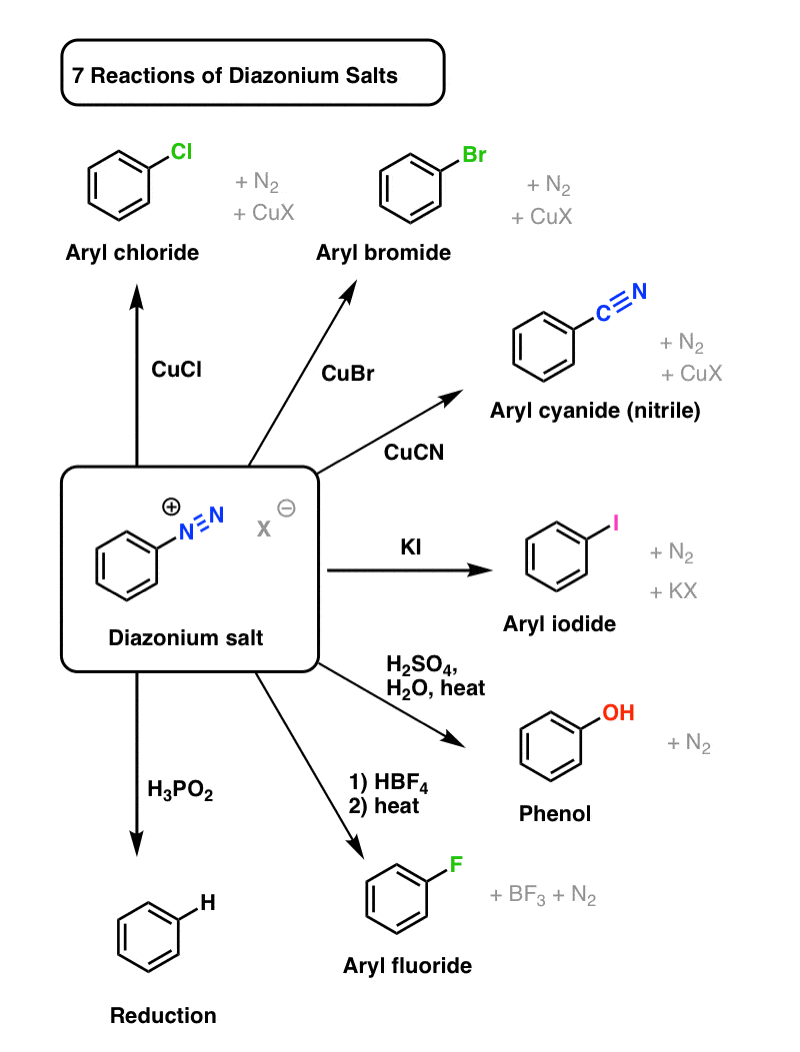
Any process featuring a single starting material that can be transformed into seven different potential products can be reasonably described as "versatile".
These reactions can be roughly divided into two categories: Sandmeyer reactions, and everything else.
Sandmeyer Reactions
One way to transform diazonium salts is by treating them with various compounds of copper. These are known asSandmeyerreactions, after Traugott Sandmeyer who first discovered the reaction in 1884 (with copper acetylide!).
Three key examples are:
- CuCl transforms aryl diazonium salts into aryl chlorides
- CuBr transforms aryl diazonium salts into aryl bromides
- CuCN transforms aryl diazonium salts into aryl cyanides (nitriles).
The mechanism, which you can read about elsewhere, likely proceeds through an aryl radical, which is oxidized to an aryl cation and then attacked by a nucleophile.
Other Reactions
Copper isn't necessary for substitution to occur if a strong enough nucleophile is present, or if the mixture is heated enough:
- Aryl iodides can also be obtained from aryl diazonium salts, through treatment with potassium iodide (KI).
- Hydroxyl groups (OH) can be installed on an aryl diazonium salt through heating with water and acid. (we've previously seen one example in John Roberts' work on arynes, which we covered here. )
- Aryl fluoridescan be installed through a two step process. The first involves exchanging the counterion (X–) on the aryl diazonium salt with the tetrafluoroborate (BF4 –) ion by treating the diazonium salt with HBF4. Then, when heated, fluorine can act as a nucleophile, displacing N2 and releasing BF3 as a byproduct.
- The diazonium salt can also bereducedto C–H, by treating the aryl diazonium salt with hypophosphorous acid (H3PO2).
Not so bad from a single functional group!
3. Mechanism: Formation of Diazonium Ions
- Formation of nitrosonium ion from HNO2
Now let's dig into how some of these reactions work.
First, let's go through formation of the diazonium salt, a process called "diazotization".
The first key reagent for this process is either sodium nitrite (NaNO2) or nitrous acid (HNO2). Sodium nitrite has the advantage of being an easily handled salt, while HNO2is a somewhat unstable liquid.
The second key reagent is a strong mineral acid like HCl; if NaNO2 is used, HCl converts it into HNO2.
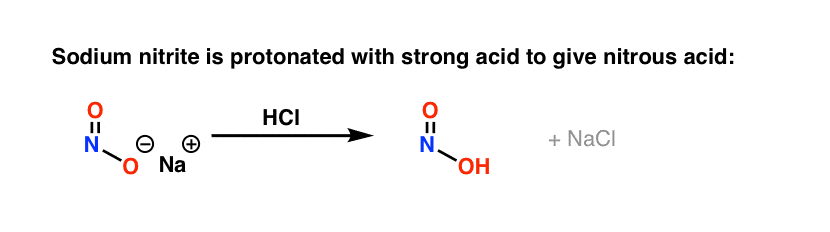
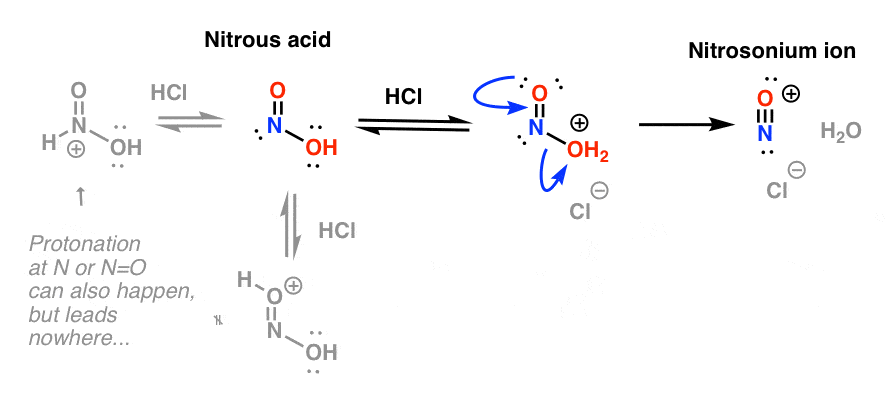
The key purpose of HCl is to further convert HNO2 into the powerful electrophile NO+, the "nitrosonium ion", which is the key electrophile in the reaction that forms the diazonium salt.
The nitrosonium ion is formed through protonation of OH and resultant loss of water:
2. Formation of the diazonium ion
The next step is formation of the diazonium ion from the reaction between the amine and the nitrosonium ion, which also requires acid.
How does it work?
The first step is formation of a new N–N bond, which occurs through attack of the nitrosonium ion by the aromatic amine (Step 1). This is followed by two proton transfers from nitrogen to oxygen (Steps 2 and 3) accompanied by reorganization of the pi bonding framework [forming N–N (pi), breaking N–O (pi) ]. The final step is formation of the nitrogen-nitrogen triple bond accompanied by expulsion of water (Step 4).
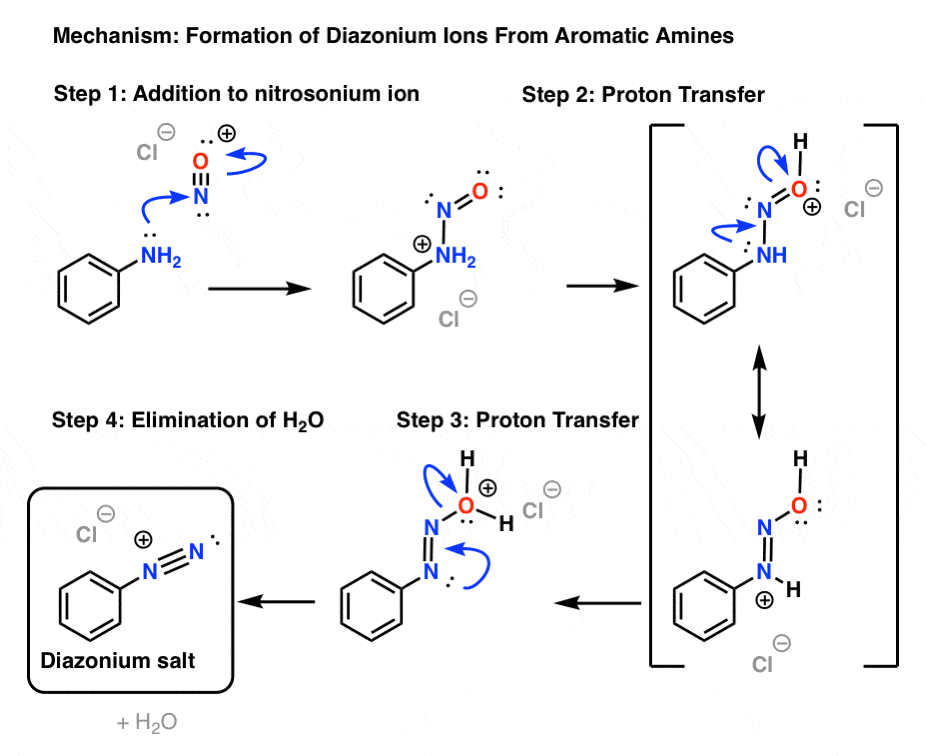
Being fairly unstable (and potentially explosive), diazonium salts are typically not isolated (it's relatively safe to handle the tetrafluoroborate salts as solids, but that's about it). Once formed, they're usually treated immediately with the appropriate reagenten route to the desired product.
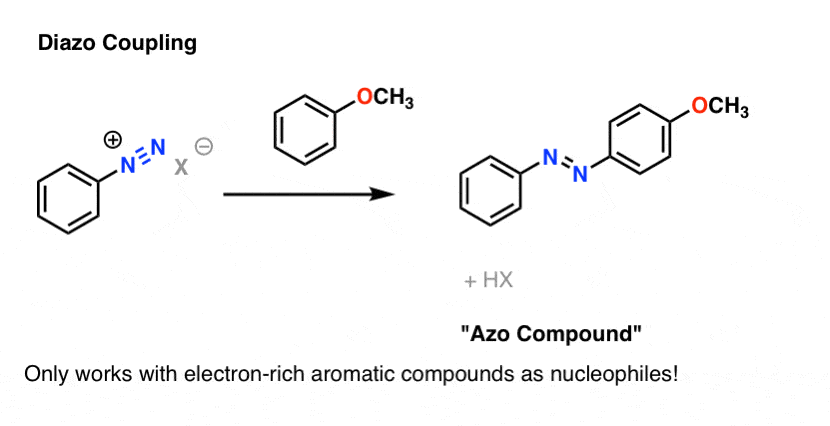
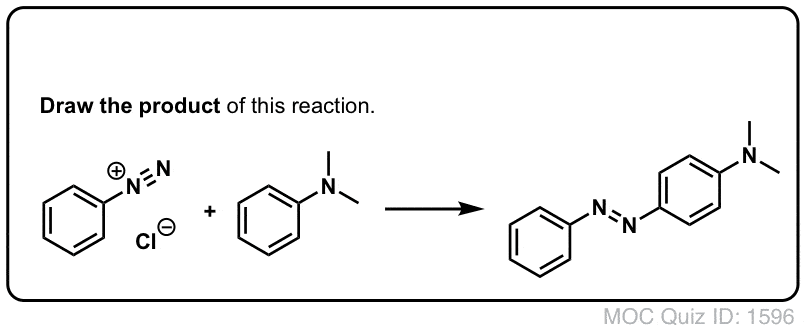
4. Bonus Reaction: Diazo Coupling
There's one last reaction of diazonium salts which is worth mentioning. A surprising number of dyes in our daily experience are derivatives ofdiazobenzene, the essential structure of which is two benzene molecules joined by a nitrogen-nitrogen double bond. See this article onazo dyes, for instance. Yellow, red, and orange are common colors of azo dyes.
Azo dyes are made through the reaction of an electron-rich aromatic partner with a diazonium salt. Only electron-rich aromatic species are good enough nucleophiles to attack diazonium salts.
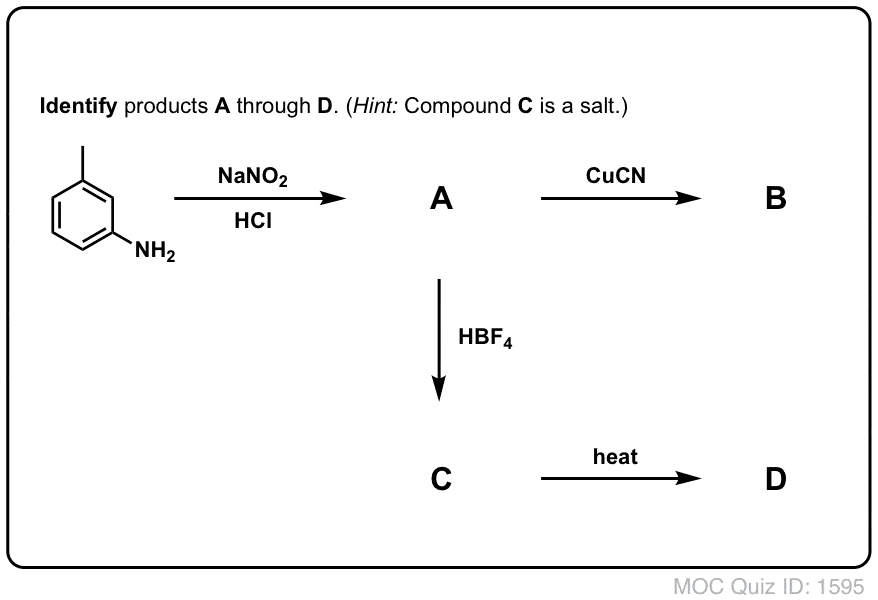
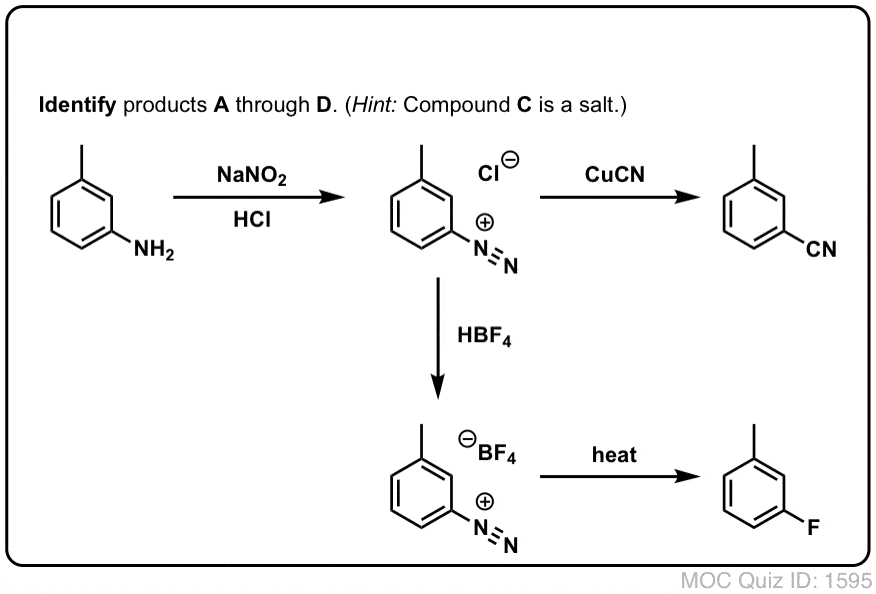
Quiz Yourself!
 Click to Flip
Click to Flip
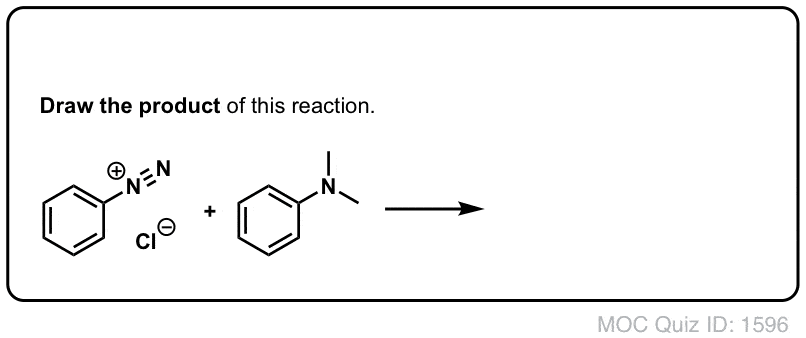 Click to Flip
Click to Flip
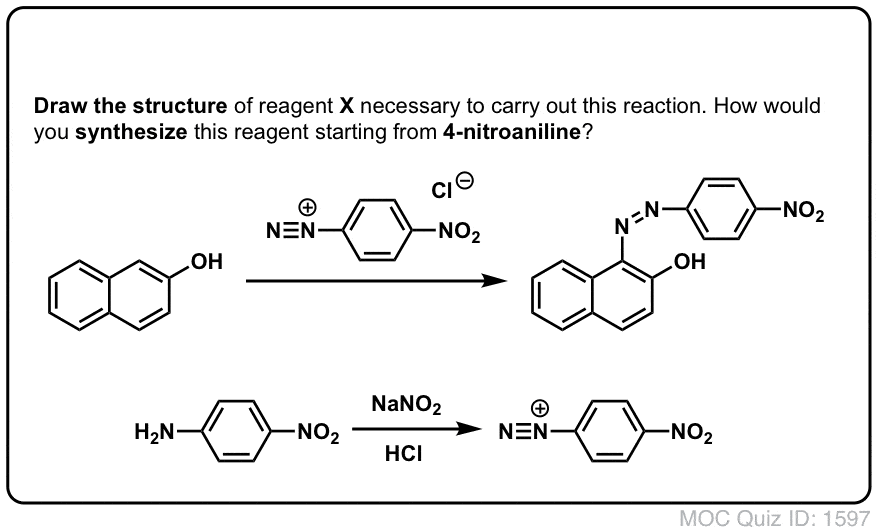
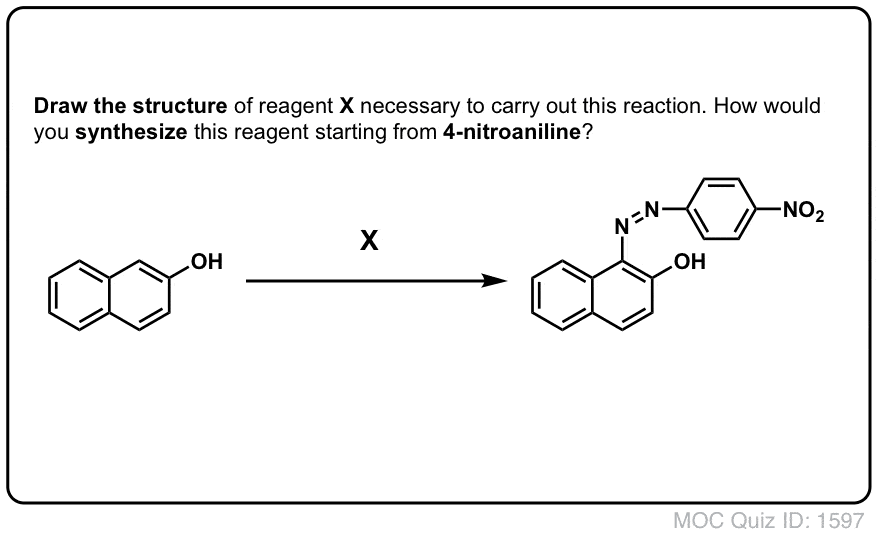 Click to Flip
Click to Flip
(Advanced) References and Further Reading
- Ueber die Ersetzung der Amidgruppe durch Chlor in den aromatischen Substanzen
Traugott Sandmeyer
Ber. 1884 17 (2), 1633-1635
DOI: 10.1002/cber.18840170219
This paper describes the accidental discovery of the Sandmeyer reaction by T. Sandmeyer. He was attempting to synthesize phenylacetylene by combining benzenediazonium chloride and cuprous acetylide, and instead obtained chlorobenzene. - Ueber die Ersetzung der Amid‐gruppe durch Chlor, Brom und Cyan in den aromatischen Substanzen
Traugott Sandmeyer
Ber. 1884 17 (2), 2650-2653
DOI: 10.1002/cber.188401702202
Sandmeyer generalizes the reaction to include the syntheses of bromobenzene and benzonitrile using CuBr and CuCN. - The Sandmeyer Reaction.
Herbert H. Hodgson
Chemical Reviews 1947, 40 (2), 251-277
DOI: 10.1021/cr60126a003
This is an old review, useful if you want to look for some of the original references for this chemistry. - Über aromatische Fluorverbindungen, I.: Ein neues Verfahren zu ihrer Darstellung
Günther Balz, Günther Schiemann
Ber. 1927, 60 (5), 1186-1190
DOI: 10.1002/cber.19270600539
The formation of aryl fluorides from the decomposition of aryldiazonium tetrafluoroborates is now known as the Balz-Schiemann reaction, after the chemists who first described the reaction. - The Mechanism of the Sandmeyer and Meerwein Reactions
Jay K. Kochi
Journal of the American Chemical Society 1957, 79 (11), 2942-2948
DOI: 10.1021/ja01568a066
Kochi was an eminent physical organic chemist in the 20th century. In this paper, he examines the mechanism of the Sandmeyer reaction, providing further proof that it proceeds through an aryl radical intermediate. - Radical reactions of arenediazonium ions: An easy entry into the chemistry of the aryl radical
Carlo Galli
Chemical Reviews 1988, 88 (5), 765-792
DOI: 10.1021/cr00087a004
This review covers the results of various investigations on the mechanism of the Sandmeyer and related reactions – these are proposed to go through aryl radical intermediates. - An investigation of the two-step nature of the Sandmeyer reaction
Carlo Galli
Chem. Soc., Perkin Trans. 2, 1981, 1459-1461
DOI: 10.1039/P29810001459
The role of Cu salts in the Sandmeyer reaction is to transfer an electron to the intermediate aryl cation, forming an aryl radical. If this is true, then other metal salts and compounds (e.g. ferrocene) should be able to reduce diazonium salts, and this is examined here. - The Hypophosphorous Acid Deamination of Diazonium Salts in Deuterium Oxide
Elliot R. Alexander and Robert E. Burge Jr.
Journal of the American Chemical Society 1950, 72 (7), 3100-3103
DOI: 10.1021/ja01163a082
While the reduction of arenediazonium salts with H3PO3 may seem like a useless reaction, this paper shows a very useful application – if you do this in D2O, you can do ipso-deuteration! - A simple preparation of phenols from diazonium ions via the generation and oxidation of aryl radicals by copper salts
Theodore Cohen, Albert G. Dietz Jr., and Jane R. Miser
The Journal of Organic Chemistry 1977, 42 (12), 2053-2058
DOI: 10.1021/jo00432a003
As the introduction of this paper states, the conversion of aryldiazonium ions to phenols is simple on paper but not necessarily in practice. High acidity is required to suppress side reactions, such as azo coupling. The use of Cu salts for this reaction as well allows cleaner reactions, higher yields, and simplified workup. - Mechanism of formation of aryl fluorides from arenediazonium fluoborates
Gardner Swain and Randall J. Rogers
Journal of the American Chemical Society 1975, 97 (4), 799-800
DOI: 10.1021/ja00837a019
An elegant kinetic study to determine the mechanism of the Balz-Schiemann reaction. Because of the insensitivity of the product distribution to excess BF3, it is proposed that the fluorobenzene is formed by direct capture from BF4 – by the Ar+ intermediate formed by dediazonization. This would be a tight ion pair in solution, and quite favorable entropically as well. - FLUOROBENZENE
T. Flood
Org. Synth. 1933, 13, 46
DOI: 10.15227/orgsyn.013.0046
A standard procedure for a Balz-Schiemann reaction. - Reactivity and stability of arenediazonium ions
Heinrich Zollinger
Accounts of Chemical Research 1973, 6 (10), 335-341
DOI: 10.1021/ar50070a002
Prof. Zollinger (ETH Zurich) was the guru of diazonium ion chemistry, and this review covers a lot of work on the various reactions of aryldiazonium ions.
complete the mechanism for an azo coupling by drawing in the curved arrows where needed.
Source: https://www.masterorganicchemistry.com/2018/12/03/reactions-of-diazonium-salts-sandmeyer-and-related-reactions/
Posted by: drakeimensid.blogspot.com

0 Response to "complete the mechanism for an azo coupling by drawing in the curved arrows where needed."
Post a Comment